What are moles?
Moles are used to count particles because particles are so unbelievably small that a huge number of them can fit in a beaker. One mole of a something is simply 6.02 1023 times of it. This number is bigger than you’d think. For a comparison this number is 10,000 times bigger than the total number of grains of sand on earth, however it is only 18 ml’s of water!
Avogadro's number: The number of particles which equal one mole - 6.02 1023
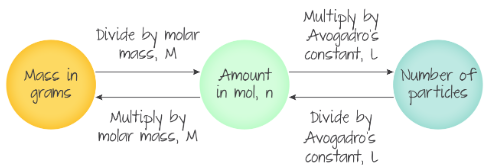
Converting between particles,moles and grams
- To convert from moles to particles multiply the number of moles by avogadro's number
- To convert from moles to grams multiply the number of moles by the molar mass(Mr)
- When going the other way divide instead of multiplying
E.g 0.2g of Al2O3in moles:
- Molar mass of Al2O3= 27*2+16*3 = 102g/mol
- Moles = 0.2/102 = 0.002 mol
E.g A sample of magnesium contains 1.41 1021calculate the number of moles:
- Moles =1.411023/6.02 1023 = 0.00234 mol
Stoichiometry
In stoichiometry you try to find the relationship between two or more substance which are part of a reaction
Mole ratio
The mole ratio is the ratio between the moles of different compounds in a chemical formula.
You can find the mole ratio by looking at the coefficients in front of the compound in the formula.
Example:
The mole ratio between the oxygen and the water is 1:2, as for every one mol of oxygen reacted, 2 moles of water is produced.
Concentration of a solution
Concentration = Moles ÷ Volume(dm3)
Units: mol/dm3
1 dm3 = 1L = 1000 cm3 = 1000ml
Example question
The equation for the reaction is:
Calculate the concentration of the sodium hydroxide, if you know the following values:
- 27.1 cm3 of sulphuric acid
- 25.0 cm3 of sodium hydroxide
- 0.100 mol/L of sulfuric acid
- Convert all volume units to dm3 :
- 27.1 cm3 = 0.0271dm3
- 25.0 cm3 = 0.0250 dm3
- Moles of sulphuric acid = 0.1*0.02712 = 0.00271 mol
- Mole ratio between sulphuric acid and sodium hydroxide is 1:2, so there are 0.00542 mol of sodium hydroxide
- Concentration = 0.00542/0.0250 = 0.217 mol/dm3
Acid-base titration
A Titration is a method of finding the concentration of an unknown solution using a solution with a known concentration.
An Acid-base titration is a type of titration where one solution is an acid and the other is a base. To find the concentration of an unknown acid/base a titration can be performed. to find the concentration of a base, you can add acid to the base using a buret until all of the base is neutralised. In order to know whether it has reached the point of neutralisation an indicator such as phenolphthalein is added which will change colour indicating the point has been reached..
Titrant: The solution with the known concentration in buret, can be an acid or a base
Analyte: The solution with the unknown concentration in conical flask, can be an acid or a base.
Buret: a graduated glass tube with a tap at one end, for delivering known volumes of a liquid in titrations.
Phenolphthalein: A ph indicator which is colourless in the presence of an acid and pink in the presence of a base. It helps determine the point of neutralisation
Procedure for an acid-base titration
Apparatus required: burette, conical flask, measuring cylinder and pipette
- Use the pipette to measure out 10 cm^3 of the analyte and pour it into the conical flask
- Add 2-3 drops of the indicator phenolphthalein to the conical flask
- Rinse the buret with distilled water and then some of the titrant
- Using a funnel in the buret fill it with the titrant. Make sure the tap is closed.
- Do a preliminary trail to figure out roughly how much of the titrant is needed to neutralize the analyte. Turn the tap to open the burete and pour the solution into the flask. Stop when the colour of the analyte solution has changed completely. Make sure to swirl the flask to make sure all the solution has neutralized.
- Do the trial for real to find the exact amount of titrant needed to neutralise the analyte. Do this by reading the change in volume on the buret.
- Repeat for two more trials, each time recording the change in titrant volume.
- Find the meam volume from the trials and do some data processing to find the concentration of the analyte.
Data-processing
Moles of titrant = Concentration of titrant * volume of titrant
Mole ratio = coefficients in front of compounds in formula
Moles of analyte = moles of titrant * mole ratio
Concentration = moles of analyte / volume of acid
View count: 7217