States of matter
There are three main states of matter which you need to know for the MYP: solid, liquid and gas. These states are dependent on the temperature of the substance.
Particle arrangement and movement
| State |
Arrangement of particles |
Movement of particles |
Diagram |
| Solid |
Close together and structured |
Only vibrate |
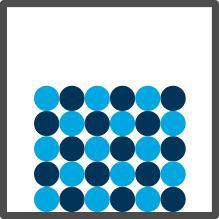 |
| Liquid |
Close together and unstructured |
Move around while still staying connected |
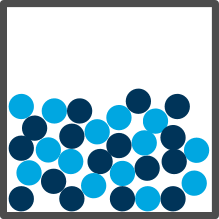 |
| Gas |
Far apart and random |
Free to go anywhere |
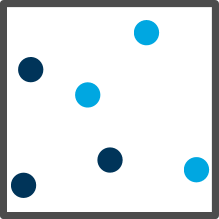 |
Shape and Compressibility
| State |
Shape |
Compressibility |
| Solid |
Fixed shape cannot flow, because the bonds between the particles prevent any movement |
Cannot be compressed as there is no space for the particles to move into as they are already tightly packed. |
| Liquid |
Takes shape of container and can flow because enough bonds are broken to allow movement |
Cannot be compressed as there is no space for the particles to move into |
| Gas |
Completely fills container and flows quickly as their are no bonds restraining it. |
Can be highly compressed as there is a lot of free space. |
Brownian motion
Definition: Brownian motion is the random motion of particles in a fluid due to continuous collisions from the molecules in the medium surrounding them.
E.g The motion of smoke particles viewed under a microscope.
View count: 4994