Electron arrangement
Electron shells
Electron shells naturally want to have a full outer shell
| Level of shell |
Number of electrons |
| 1st level |
2 |
| 2nd level |
8 |
| 3rd level |
8 |

Relationship between electron shells and the periodic table
- The number of electrons in the outer shell is equal to the group number
- The number of shells is equal to the period.

Ions
Ions are elements which have had electrons added or removed, giving them a charge.
Cation vs Anion
Cation: An ion with less electrons than normally. It has a positive charge .
Anion: An ion with more electrons than normally. It has a negative charge.
Bonding
Types of bonding
| Bonding name |
Type of elements |
Diagram |
| Covalent |
Simple molecular |
Non-metal and Non-metal |
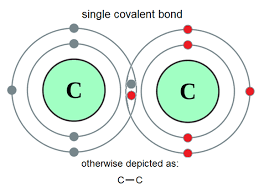 |
| Giant molecular |
Carbon or Silicon with itself. |
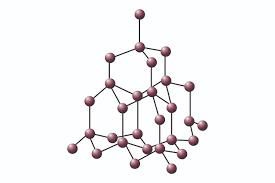 |
| Metallic |
Metal and Metal |
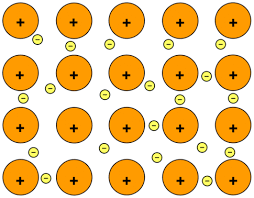 |
| Ionic |
Non-metal and metal |
 |
Lewis structures
A lewis structure is a diagram specifically for Covalent bonding Which shows which electrons are shared in the bond of a molecule.
How to draw it:
- Start by drawing all the electrons as crosses around an element
- Then circle electron pairs to show that they are shared across the elements.
- Circle the electrons so that they add up to 8; a full shell.
- Neaten it up by replacing any pairs of electrons with a single line
Example diagram
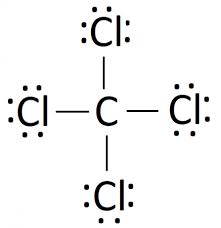
View count: 8672