Atoms And Molecules
Avogadro's constant
6.02x1023. This is the number of molecules in 12g of carbon 12. It is also the number of molecules in exactly one mole of atoms.
Mole
This is the standard unit of measurement for "amount of substance." One mole of a substance contains 6.02 x 1023 molecules.
Molar mass:
The weight of one mole of an element. This can be found above an element symbol on the periodic table.
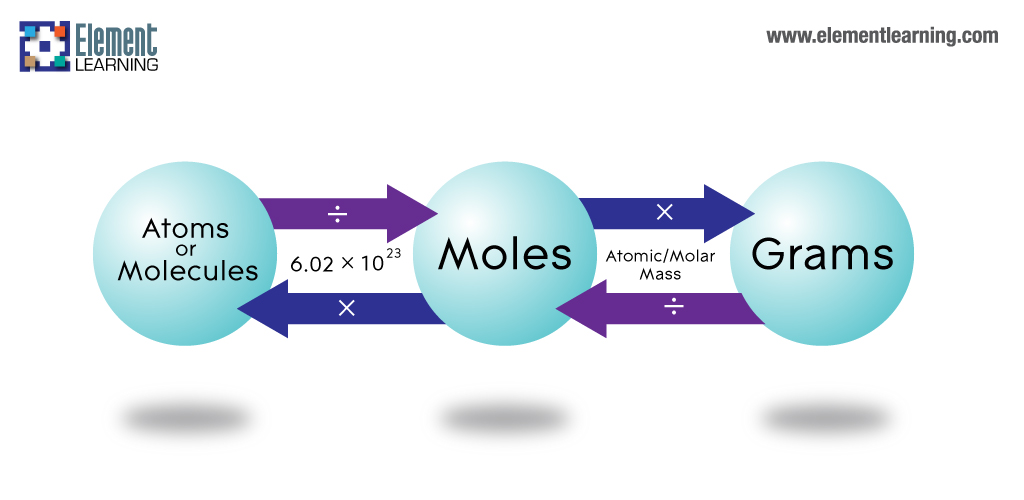
Conversion between molecules, mass, and moles
- Convert from the number of molecules to moles by dividing by 6.02x1023
- Convert from moles to grams by multiplying by the molar mass
Kinetic theory of matter
The kinetic molecular theory of matter states that: matter is made up of particles that are continually moving. All particles have energy, but the energy varies depending on the temperature of the matter.
Brownian motion is the constant erratic movement of small particles suspended in a liquid or gas when viewed under a microscope. This is caused by the constant bombardment from the molecules in the medium. This provides evidence for the kinetic theory of matter.
Thermal Concepts
Internal energy
Internal Energy is the sum of all random kinetic energies and mutual potential energies of the particles of the body or system. It is measured in joules (J).
Average Kinetic energy: The average amount of energy stored in each particle due to its motion. The faster it vibrates or moves, the more kinetic energy
Potential Energy: The energy required to stretch the intermolecular bonds.
Temperature
This is a measure of how hot or cold a substance is. More specifically, it is a measure of the random average kinetic energy. It is measured in °C or K.
The average kinetic energy of a molecule is given by this equation (in data booklet):
- k is the Boltzmann constant
- T is the temperature in Kelvin
Heat
Heat is the transfer of energy from a high temperature to a low temperature. The transfer occurs until both objects are at Thermal equilibrium
Heat transfer
Conduction: When the molecules at one end of a solid object are given energy, they vibrate more. This disturbs the neighboring molecules and passing the energy along.
Convection: The transfer of heat energy via liquid or gas. When heated fluid expands, marking it less dense, causing it to rise in the surrounding denser cooler fluid.
Radiation is the direct transfer from one body to another via infrared radiation. Bodies of darker color both radiate and absorb the best.
Thermal capacity
The heat required to raise the temperature of a body by 1k.
Formula:
Unlike specific heat capacity, this does not require mass.
Specific heat capacity
Specific heat capacity: Amount of energy (joules) required to raise 1kg of a substance by one degree (Kelvin or Celcius). Measured in J/kg°K
Formula: $$c=Q/{mΔT}$$
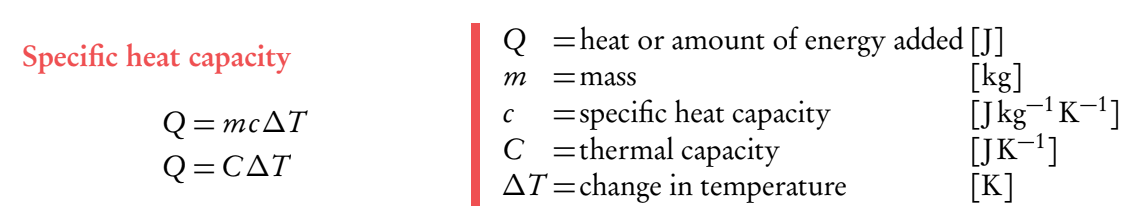
The change in thermal energy is equal to mass times specific heat capacity times by change in temperature.
Example question and answer
Jacob has 1500g of water, which is at 17°C. How much heat must he add in joules, for the water to begin to boil? The specific heat capacity of water is 4.2 KJ/kg°C.
- First, pull out the values needed from the question:
- Change temperature = 100-17= 83°C
- Mass = 1500g = 1.5kg
- SHC = 4.2 KJ/g°C.= 4200j
- Then put into the formula:
Specific latent heat
Specific Latent Heat: Energy required to change the state of 1 kg of a substance. The unit is j/kg
When you change the state of a substance, the energy stops being used to change the temperature but instead to break the bonds. Because of this, during a period of state change, the temperature will remain constant.
The amount of energy required to change the state of a substance is called the latent heat. Latent is a word which means that something exists yet is hidden, so this is the "hidden temperature" change.
Fusion and vaporization
Since there are two state changes, there are also two types of specific latent heat
Latent heat of fusion: Energy (kJ) to change a solid into a liquid
Latent heat of vaporization: Energy (kJ) to turn a fluid into a gas.
Calculating Latent heat changes

The energy to change the state is equal to mass times specific latent heat for that state.

Modeling An Ideal Gas
Assumptions of an ideal gas
- The volume of atoms is much smaller than the volume of gas
- Atoms are perfectly elastic spheres. (they don't lose energy in collisions)
- There are no intermolecular forces between the gas molecules.
- There is no potential energy as this requires inter-molecular forces
- The collisions all have uniform velocity
- High temperatures and low pressures
The IB will usually only ask for three assumptions, so if you remember these you have a very high chance of getting full points on any relevant question.


Ideal gas law
- P-pressure (pa)
- v-volume ()
- n-moles
- r - gas constant (8.31)
- t - temperature (kelvin
Boyles law
- The pressure of a fixed mass of gas at constant temperature is inversely proportional to its volume
- The pressure of a fixed mass of gas at constant volume is proportional to its temperature
- The volume of a fixed mass of gas at constant pressure is proportional to its temperature.
Types of processes
- Isobaric -constant pressure
- Isovolumetric - constant volume
- Isothermal - constant temperature
- Isolated - constant mass
Thermodynamic cycle: A set of operations which ultimately return to its original state
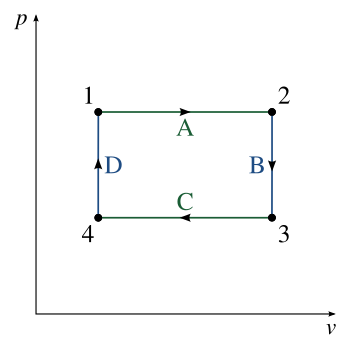
The Mother of all Physics Questions
This really is the hardest multi-choice question on this topic.
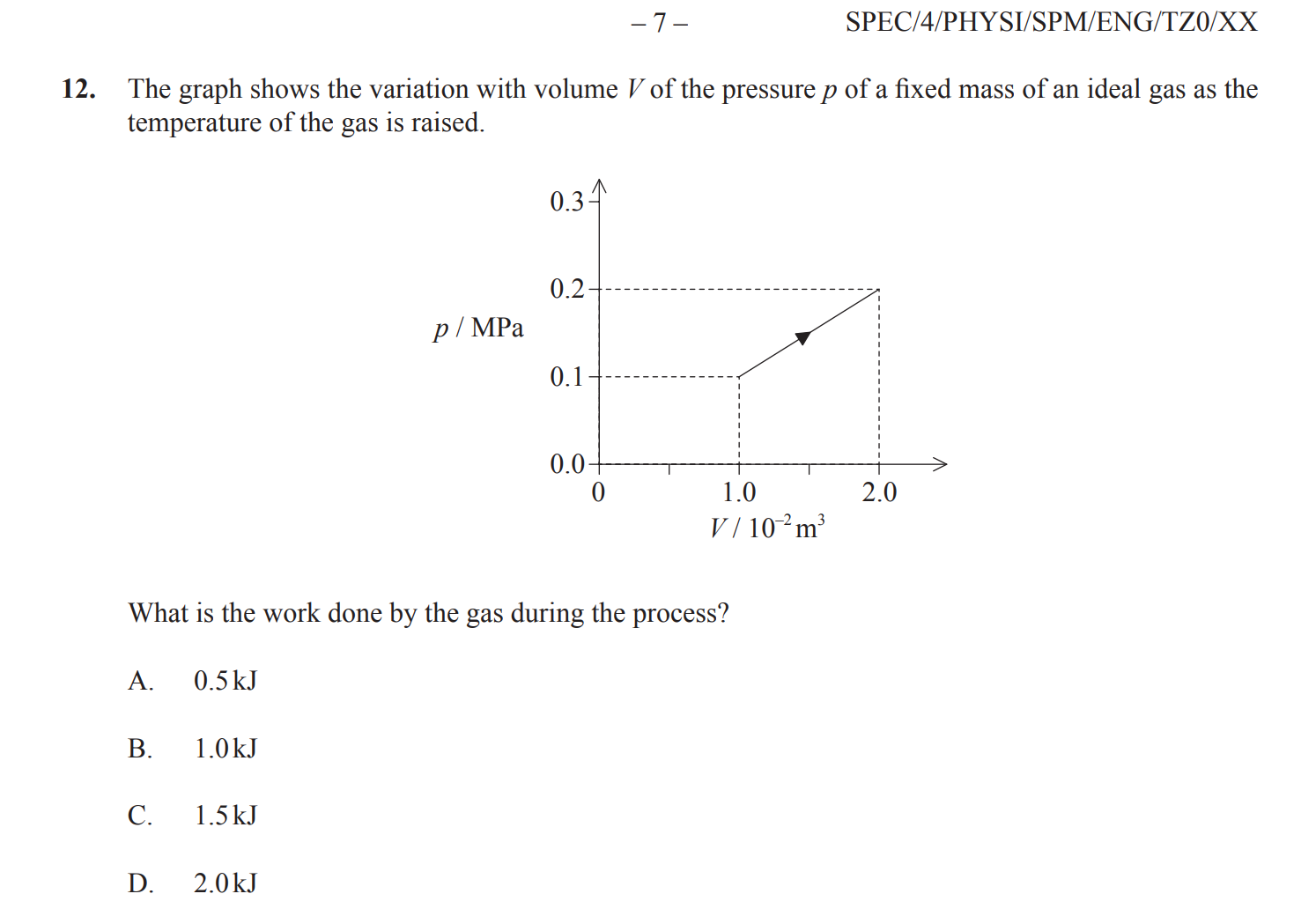
A: C (W=f*d and f = A * P therefore W = P * change in V )
Editors- joeClinton - 890 words.
- CD_FER - 132 words.
View count: 10493