Oxidation and reduction
Definitions of oxidation and reduction
| Definition type |
Oxidation |
Reduction |
| Traditional |
Gain of oxygen |
Loss of hydrogen |
| Electron transfer |
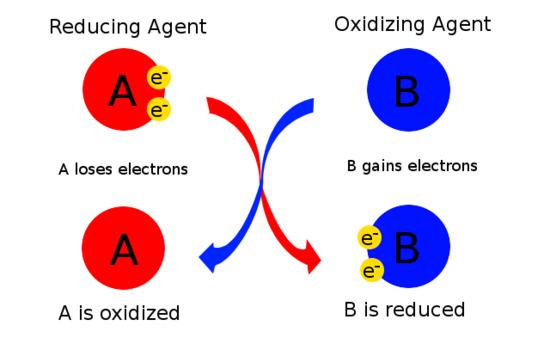
Loss of electrons |
Gain of electrons |
| Oxidation state |
Increase in oxidation state |
Decrease in oxidation state |
use OILRIG to memorize
Oxidizing and Reducing agents
Oxidizing agent: a compound which oxidizes other substances, getting reduced in the process
Reducing agent: a compound which reduces other substances, getting oxidized in the process.
Oxidation states
An oxidation state describes the degree of oxidation of an atom in a chemical compound. it can be though of the hypothetical charge the atom would have if the bond was completely ionic.
Example
in the oxidation state of oxygen is +2 because in our hypothetical "all bonds are ionic" world the oxygen loses two electrons to the hydrogens.
Things to beware of
- The oxidation sign should be placed before the oxidation number e.g +3 not 3+
- The oxidation state and number are often used interchangeably but they are slightly different. The number is often represented in roman numerals and does not include the sign, it is more commonly used with the transition metals.
Rules for determining oxidation states
In Order to determine the oxidation state of an atom, there are some important rules which have to be memorized. These are summarized in following list of rules, where each rule can be broken by an earlier rule.
- The total oxidation state of a molecule equals the overall charge. (so in a neutral molecule the sum of oxidation states is 0)
- Free elements (e.g H2, Fe, O2) are always 0
- The total oxidation state of polyatomic ions equals the charge
- Ions in compounds always have an oxidation state equal to their charge.
- Fluorine has an oxidation state of -1
- Oxygen has an oxidation state of -2 (except in peroxides e.g. H2O2, where it is −1)
- Group 1 molecules have an oxidation state of +1
- Group 2 molecules have an oxidation state of +2
- Hydrogen has an oxidation state of +1 (except in certain metal hydrides e.g. NaH, where it is −1)
- Halogens (e.g Cl, Br, I) in compounds are assigned an oxidation state of −1. (Chlorine may vary when with oxygen or fluorine)
Reasons for exceptions
- Hydrogen is -1 in a metal hydride because it has to balance with the positively charged metal.
- Oxygen is -1 in peroxides because these molecules are neutral and simply it wouldn't be neutral if this wasn't the case.
- Chlorine when with fluorine or bromine can have lots of different oxidation states so work it out after all the rest.
Redox reactions
Balancing half equations
After splitting a redox reaction into two half equations there are a series of steps that we need to use to balance half equations.
The example is
| # |
What to do |
Example |
|
1
|
Balance the atom being oxidized/reduced, by multiplying either side
|
The right side is multiplied by two so that there are two Cr on either side
|
| 2 |
Balance the oxygens by adding H2O.
(We use water not oxygen because in an aqueous solution water is present but not free oxygen)
|
There are 7 oxygen on the left so 7 H2O's are added
|
| 3 |
Balance the hydrogens by adding H+ ions |
The 7 H2O's on the right means there are 14H so 14 is need to balance it.
|
| 4 |
Balance the charge by adding electrons to the more positive side |
(14-2)-(2x3) = 6, so there needs to be 6 electrons added on the left
|
Balancing redox reactions
Use the following step to balance a redox reaction
- First figure out what is being oxidized and what is being reduced, then based on this split into two half equations
- Balance each half equation
- Balance the electrons gained/lost for both equations by multiplying everything by a constant
- Recombine half equations
Redox titrations
A redox titration is a titration which involves a redox reaction between a titrant and analyte.
Steps and example
Question: a tablet of Iron(II) sulphate dissolved in acid is titrated with 22.4 cm3 of 0.100 mol dm-3 potassium manganate (VII) solution, what is the mass of iron in the tablet?
Note: Although unspecified you are expected to know that the acid the titrant is dissolved in, should not be reduced or oxidize itself. E.g sulphuric acid for Iron(II) sulphate
| Step |
Example |
| Determine mols of titrant. Multiply concentration by volume. |
|
| Determine chemical formula of titrant and analyte. |
- Iron(II) sulphate = FeSO4
- potassium manganate (VII) = KMnO4
|
| Determine which is oxidized and which is reduced. (Normally, the titrant is reduced) |
- FeSO4 is oxidized
- KMnO4 is reduced
|
|
Split compounds into ions, then determine the unbalanced half equations.
|
|
| Balance half equations |
|
| Combine in correct ratios to make full redox reaction. Then add spectator ions back. |
|
| Find ratio between analyte and titrant |
5:1 |
| Mass of analyte = Molar mass of analyte x mols of titrant x mol ratio |
|
Winkler's method
This is used to measure the biochemical oxygen demand (BOD) in the water, which is used as a measure of pollution in a water sample.
Biochemical oxygen demand (BOD): The amount of oxygen which is required to be dissolved in the water in order for bacteria in the water to be able to decompose organic waste in 5 days.
| Step |
Chemical equation |
|
Saturate water sample with oxygen, so that initial concentration of dissolved oxygen is known. Then incubate the sample for 5 days. This allows the bacteria to deplete the oxygen, while decomposing organic matter.
|
|
| After 5 days remove the sample and then to prevent the oxygen amount changing any more Manganese sulphate is added.This reacts with the dissolved oxygen to form manganese (II) oxide precipitate. |
|
| The manganese oxide is then oxidized with an excess of potassium iodide. The iodide ions are oxidized to form iodine. The reason we do this step is because it is easier to measure Iodine than manganese oxide. |
|
|
The amount Iodine produced is measured through a redox titration with Sodium thiosulphate.
Starch is used as an indicator because in the presence of iodine the water is blue, and colourless when not in the presence.
|
|
- the # of moles of iodine is half the moles of S4O6
- The # of moles of Manganese oxide is the same as the # of moles of iodine
- The # of moles of oxygen is half the moles of Manganese oxide
So the mole ratio between dissolved oxygen and S4O6 is 1:4
|
|
Rather than doing the whole process in some questions you can just use the 1:4 mole ratio.
Voltaic and electrolytic cells
Voltaic cells
Voltaic cells use spontaneous redox reactions to produce an electric current. They consist of two half cells connected by a salt bridge.
Half-cell: This is a (metal) electrode resting in a solution of its own ions. E.g a copper half cell, consists of a copper electrode in a solution of copper ions. It must be the same to prevent side reactions.
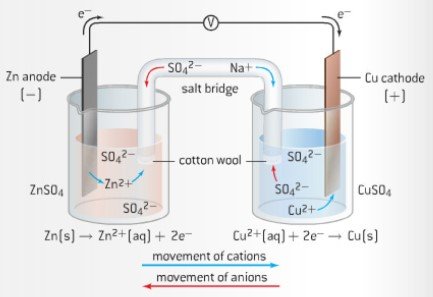
Diagram taken from IBchem.com
How do voltaic cells generate current?
In the voltaic cell, the current comes from the potential difference between the electrodes. Therefore to get the highest potential difference we want a very reactive element and an unreactive element. The difference can be seen by looking at the activity series.
Normally, both electrodes probably would choose to oxidize, however when the half cells are connected by a salt bridge, only one of the two can oxidize. This is the more reactive electrode. The other electrode will be forced to reduce and accept the more reactive electrodes ions.
The voltaic cell cycle
For the following example we will use the same set up as in the above diagram. With a zinc anode and a copper cathode.
- The Zinc anode being more reactive than the copper cathode will oxidize and push it's electrons onto the wire it is connected to.
- The electrons will flow down the wire providing current to the load before arriving in the cathode. Here it will reduce the cathode
- The cathode gains more electrons thus, it is now negatively charged and the cations in the electrolytic solution will now be attracted to the cathode and stick to it. They come out of solution and can be visibly seen.
- On the other side the oxidized Zinc ions, leave the anode and become part of the electrolyte
- There is now an imbalance of charge, which is solved by adding a salt bridge which allows the ions to beg given to each side in order to balance the charge
Electrolytic cells
An electrolytic cell is where an an electric potential is applied to an aqueous solution, in order to split up the ions and extract the elements.
The electrodes are inert and made of carbon

Anode vs Cathode charge
- Anode = positive: The anode oxidizes the negative ions, therefore it must take its electrons. Therefore it is positively charged
- Cathode = negative: The cathode reduces the positive ions, therefore it must give electrons. Therefore it has a negative charge.
What will be the product of the electrolysis in aqueous solution
Here there is also the dissociation of water which will produce hydrogen ions and OH ions.
If we also dissolved Sodium chloride then at either the cathode or anode there is competition betweenthe ions. The one which wins depends on the position in the activity series.
The hydrogen ions are reduced as they are lower in the scale.
The chloride ions are oxidized as they are higher in the scale.
Comparison of electrolytic and voltaic cells
| Voltaic cell |
Electrolytic cell |
| Oxidation at anode, Reduction at cathode |
| Anode is negative, Cathode is positive. |
Anode is positive, Cathode is negative |
| Spontaneous and exothermic |
Non-spontaneous and endothermic |
| Convert chemical energy to electrical energy |
Convert electrical energy to chemical energy |
| Two separate aqueous solutions connected by salt bridge |
Electrolyte is a molten liquid or an aqueous solution |
View count: 14846