The structure of the periodic table
Periods: Horizontal rows of the periodic table
Groups: Vertical columns of the periodic table
Periodic table basics
- There are 18 groups, numbered 1-18
- The elements are ordered by increasing atomic number
- The period number (n) is the outer energy level that is occupied by electrons.
- Elements in the same group have the same number of outer electrons and have similar chemical properties
- The periodic table can be classified as non-metals, metals and metalloids
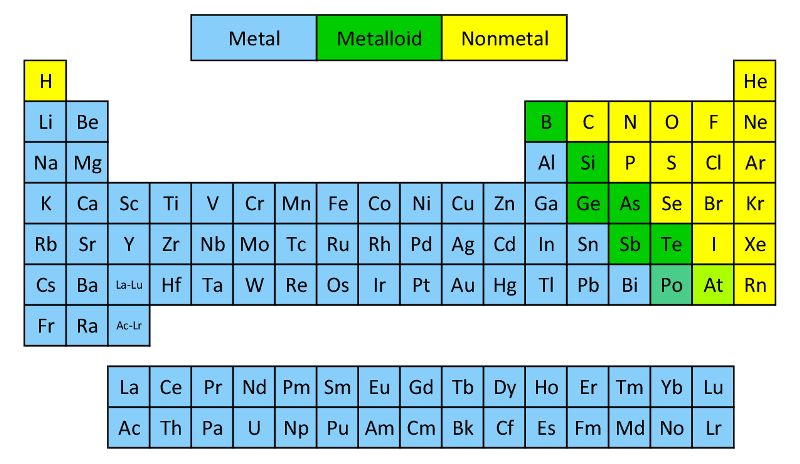
Metals, non-metals and metalloids
These different types of elements are found in different regions of the periodic table.
The periodic table and electron configuration
The periodic table is separated into different blocks. The four needed for IB are the s,p,d,f blocks.
These blocks represent the highest sublevel occupied by the elements within these blocks. The position within the block tells you the number of electrons int he orbital
- E.g Phosphorus is in the third element in the P block. So it's highest sub level in its electron configuration is P and it is filled with three electrons.
/ecblocks-56a129535f9b58b7d0bc9f2e.jpg)
The diagram above shows you how the sp,p,d,f blocks are organized. It is quite simple and easy to remember.
- Left 2 groups are the S blocks
- Right 6 group are the p blocks
- Middle 10 groups are the d blocks
- Bottom 2 rows are the f blocks. (Lanthanoids and actinoids)
The key take away is, that just by looking at where an element is positioned you can immediately know what it's configuration is.
Practise Question (V3 Question Bank):
Carbon and silicon belong to the same group of the periodic table.
(a) Distinguish between the terms group and period in terms of electron arrangement.
........................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
A: (2 Marks) Group: number of outershell/valence electrons;
Period: number of occupied (electron) shells;
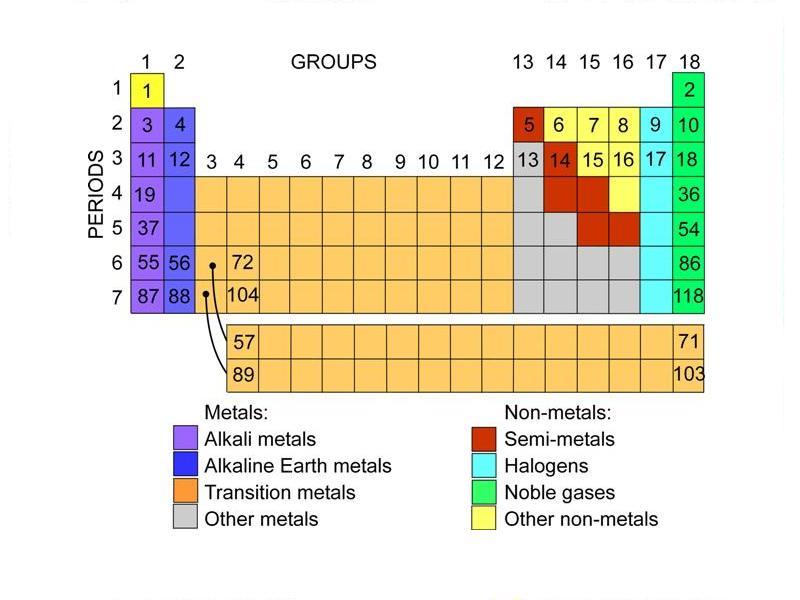
Names of special groups with characteristic properties
| Group Name |
Group number |
Characteristics |
| The alkali metals |
1 |
The most reactive group of metals – react strongly with cold water (Videos). |
| The alkaline earth metals |
2 |
Reactive metals – but less reactive than group 1. |
| The Halogens |
17 |
The most reactive group of non-metals. |
| The noble gases |
18 |
A group of very unreactive gases. |
|
Transition metals
|
The d-block region (This excludes zinc and the elements below it as they have a full d subshell)
|
Relatively stable metals with only moderate reactivity – useful for construction. |
Properties of elements and their trends
Just a note that all the trends are written about in here are in the data booklet provided in the exams but you will still need to know why these trends happen.
Atomic Radii
Definition:
- The atomic radius is not how big the atom is but is measured as half the distance between neighbouring nuclei.
- As you go from left to right, the effective nuclear charge increases because as you are adding protons, you are still in the same energy level so the inner electrons are not increasing.
Trend
- The atomic radius increases as you go down a Group because you are adding energy levels.
- The atomic radius decreases across a Period because the effective nuclear charge increases which increase the attraction of the nucleus and the outer shell electrons.

Practise Question (V3 Question Bank):
Which property generally decreases across period 3?
A. Atomic number
B. Electronegativity
C. Atomic radius
D. First ionization energy
A:C (1 Mark)
Ionic Radii
Definition
Ionic radius is the atomic radius of an ion in ionic crystal structures.
Trend
- Positive ions (cations) have smaller atomic radii than their parent atoms because they have lost electrons.
- Negative ions (anions) have larger atomic radii than their parent atoms because they have gained electrons. Increasing electron repulsion also causes the radius to increase.
- As you move across a period the ionic radii decrease with a jump between positive and negative ions.
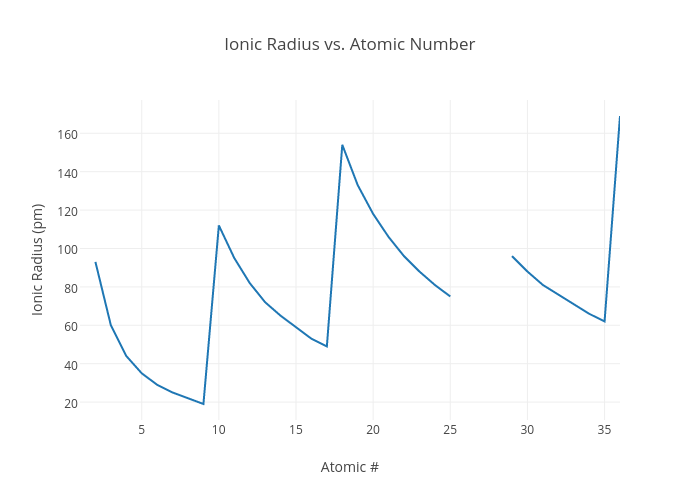
- The ionic radii increase as you move down a Group due to increasing energy levels.
Example Question (V3 Question bank)
Which species has the largest radius?
A. Cl–
B. K
C. Na+
D. K+
A: B (1 mark)
First ionisation energy
Definition
The first ionization energy of an element is the energy required to remove one mole of electrons from one mole of gaseous atoms.
Trend
- Ionization energies increase across a period due to increasing nuclear charge which makes it more difficult to remove the outer shell electron.
- Ionization energies decrease down a group. This is due to the distance from the nucleus and the shielding by the inner shell electrons.
- The small dips in the general trend across the period are caused by the sub-orbitals.
- The d-orbital fills one electron in each orbital first then they fill the top level after. Removing the bottom level requires more energy than the top

Practise Question (V3 Question Bank):
Explain why the first ionization energy of magnesium is higher than that of sodium.
................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................
A: (2 Marks) greater positive charge on nucleus / greater number of protons / greater core charge;
greater attraction by Mg nucleus for electrons (in the same shell) / smaller atomic radius;
Electron Affinity
Definition
Electron affinity is similar to Ionization energy. Electron affinity is the energy change when one mole of electrons is added to one mole of gaseous atoms to form one mole of gaseous ions.
Trend
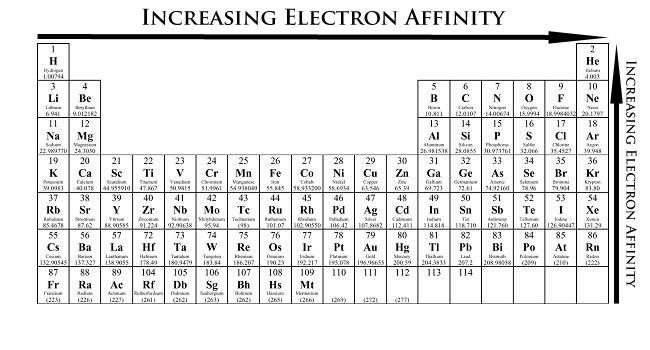
Practise Question (V4 Question Bank):
Which equation represents the first electron affinity of chlorine?
A. Cl(g)+e-→ Cl-(g)
B. 1/2Cl2(g) + e- → Cl-(g)
C. Cl+(g) + e- → Cl(g)
D. Cl(g) → Cl+(g) + e-
A:A (1 Mark)
Electronegativity
Definition
Electronegativity is the measure of the tendency of an atom in a molecule to attract a shared pair of electrons towards itself.
Trend
- Increases across period - effective nuclear charge increases pulling electron pair more.
- Decreases down the group - Electrons are further from the nucleus

Metallic character
Metals are characterised by having low ionisation energies, which means that they lose their valence electrons relatively easily to form positive ions.
Trends in metallic character
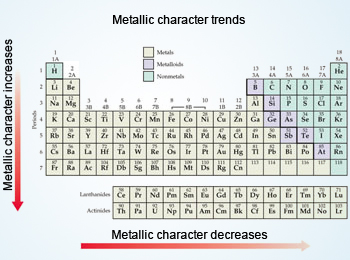
- Decreases across period - due to the increase in ionisation energy across a period.
- Increase down the group - the ionisation energy decreases.
Trends in oxide behaviour
Metal oxide: A metal bonded with oxygen
Metal oxides reacting with water
An oxide is very strongly basic ion and due to small size and high charge it forms hydroxide ions when it reacts with water.
What determines the acidity/basic of an oxide
- More electropositive central atom = More basic
- More electronegative central atom = More acidic

Trends in melting point
The melting point of a substance depends on its structure and bonding. Across a period, the structure and bonding gradually change from metallic to giant covalent to molecular covalent.
The melting point reaches a peak with silicon, which has the highest melting point because of its giant covalent structure.
To the right of silicon the atoms exist as relatively small molecules like chlorine as Cl2 molecules. Although the covalent bonds within each molecule are strong, there are only relatively weak intermolecular forces between the molecules.
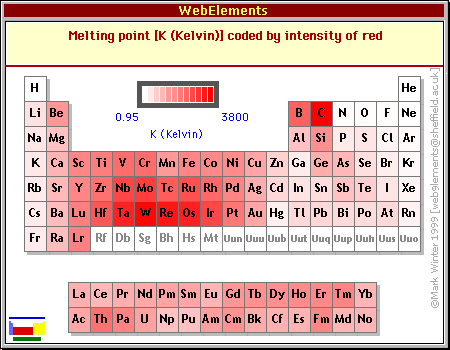
A periodic Table with all boiling and melting points is available in your exam data booklet.
Trends within groups
The alkali metals
The alkali metals are located in group 1 of the periodic table. They are a very reactive group of metals with properties quite different from those like iron and copper.
Trends of alkali metals
The melting and boiling points of the alkali metals are also relatively low and decrease down the group. This is due to the metallic bond getting weaker as the ionic radii of the metals increase.
| Group 1 metal |
Melting point (oC) |
| Lithium |
181 |
| Sodium |
98.8 |
| Potassium |
63.4 |
| Rubidium |
39.3 |
| Caesium |
28.4 |
Alkali metals undergo vigorous reactions with water to form a metal hydroxide and hydrogen gas as shown below:
2Na (s) + 2H2O (l) → 2NaOH (aq) + H2 (g)
The Halogens
The elements in group 17, known as the halogens, are a very reactive group of non-metal elements.
Trends of halogens
In contrast to the alkali metals, the reactivity of the halogens decreases down the group. Fluorine is the most reactive halogen.
| Halogen |
Melting point (oC) |
Boiling point (oC) |
State at room temperature (25oC) |
| Fluorine (F2) |
−220 |
−188 |
Gas |
| Chlorine (Cl2) |
−101 |
−35 |
Gas |
| Bromine (Br2) |
−7 |
59 |
Liquid |
| Iodine (I2) |
114 |
184 |
Solid |
The halogens can also undergo displacement reactions with each other. Chlorine will displace those halogens beneath it to the following equation:
Cl2 (aq) + 2KI (aq) → 2KCl (aq) + I2 (aq)
Properties of elements (Table)
|
Property
|
Definition |
Trend |
Diagram |
| Effective nuclear charge |
The charge felt by the valence electrons.
Effective Charge = Nucleus charge - inner electron charge
Depends on:
- Distance between valence electron and nucleus
- Number of shielding inner electrons
- The number of protons. in nucleus
|
- Decreases down the group - The number of shielding electrons increases but so does the distance between valence electrons and nucleus, which is more important.
- Increases down period - The number of shielding electrons is constant but the number protons increases
|
 |
| First ionisation energy |
The energy required to remove one mole of electrons from one mole of gaseous atoms from its valence shell
|
- Follows the trend of the effective nuclear charge
- The small dips in the general trend across the period are caused by the sub-orbitals. The d-orbital fills one electron in each orbital first then they fill the top level after. Removing the bottom level requires more energy than the top.
|
 |
| Atomic radius |
Distance from centre to the outermost orbital of electrons.
- In a covalent bond, it is half the distance between the two nuclei
- In a metallic bond, it is half the distance between two neighbouring nuclei
|
- Increases down the group - Another subshell is added
- Decreases across period - Effective nuclear charge increases so electrons are pulled closer
|
 |
| Ionic radius |
- For ions, which have a full outer shell by either losing or gaining electrons.
- Distance from centre of the ion, to the boundary of its electron shell.
- Half the distance between two ions which barely touch each other.
|
- A similar trend to the atomic radius for the same reasons.
- Groups 1-14 form positive ions by losing an outer shell while groups 14-18 form negative ions by filling the outer shell. Because groups 1-14 ions have one shell less than groups 14-18 they have a smaller ionic radius
- If all else remains the same, adding electrons to a shell increases the repulsion force increasing the ionic radius
|
 |
| Electron negativity |
The attraction of an atom for a bonding pair of electrons.
|
- Increases across period - effective nuclear charge increases pulling electron pair more.
- Decreases down the group - Electrons are further from the nucleus
|
 |
| Electron affinity |
The energy change when one mole of electrons is added to one mole of gaseous atoms to form one mole of gaseous ions.
|
- Increases in energy released across period in general, the more electronegative the element, the more it wants the electron, the larger energy released when it receives it.
- Decreases down group - further from nucleus means less attraction on electron, so less energy is released.
|
 |
Editors- joeClinton - 756 words.
- CD_FER - 1057 words.
- pdumnoenchanvanit - 68 words.
View count: 44850