Reaction rates
How is the rate of a reaction measured?
The rate of reaction is measured as a change in concentration, over the change in time. The units are the units for concentration, per unit time. So moldm-3s-1.
How do particles react?
The conditions for particles to react are:
- Sufficient energy
- Correct orientation
How does a catalyst work?
A catalyst works by reducing the activation energy of the collision.
It does this by proving an alternate pathway for the reaction to take which requires less energy.
Ways of speeding up a chemical reaction
- Catalyst
- Reduces activation energy by providing an alternate pathway
- Increase temperature
- Makes the particles move faster
- Increases frequency of collisions
- Increases collision energy
- Increase surface area (for solids)
- More surface increases the frequency of reactions
- increase pressure (for gases)
- Decreases volume which means concentration increases
- Increase concentration (for solutions)
- Increases frequency of collisions
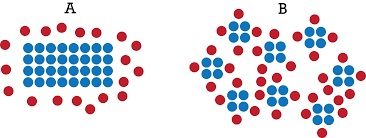
Why does temperature increase reaction rate so much?
Increasing the temperature increases the kinetic energy. An increase in kinetic energy increases both the collision energy and the collision frequency. Increasing the collision energy means that more with energy greater than the activation energy will occur. Increasing the collision frequency means a greater probability of reaction between particles.
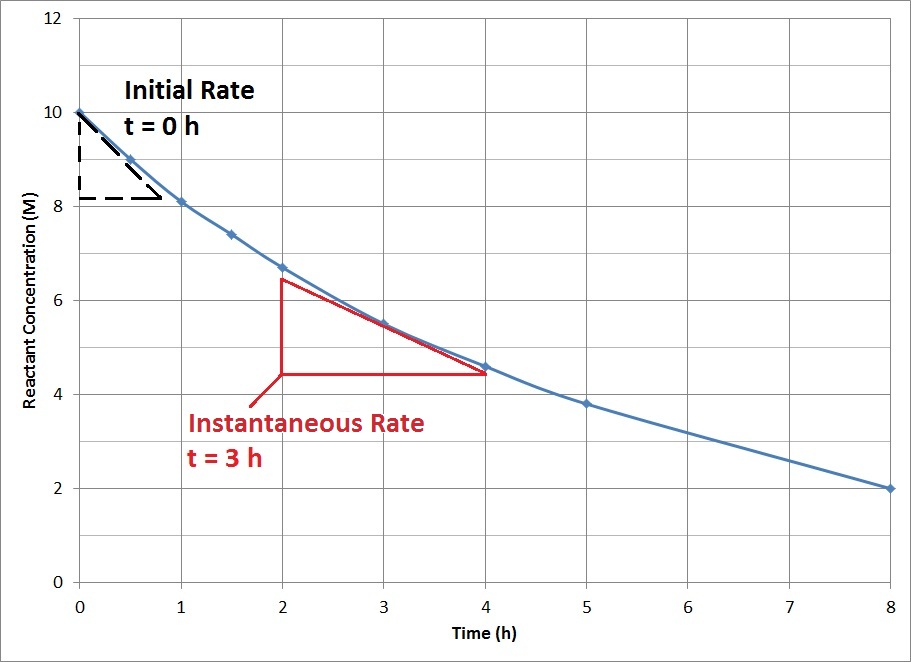
How does the rate of reaction change as the reaction progresses, and why?
In a theoretical reaction where every single variable that affects reaction rate (aside concentration) is controlled, then the initial reaction rate (the moment the reaction starts fully) will always be greater than the reaction rate at any other point during the experiment.
So Ri>Rm, where Ri is the initial reaction rate, and Rm is the reaction rate at any given moment.
The reason for this is that as the reaction progresses, the concentration of the reactants decreases. A lower concentration means a lower probability of collision, which means a low reaction rate. So the rate of reaction decreases until it eventually becomes 0, at which point the concentration is 0, as there are no reactants left.
This is shown in the graph below. (Note that the derivative of this curve is the rate of the reaction. This makes sense. If you draw a tangent line, it has a gradient :
where C is concentration, and t is time. With dimensional analysis, we see the units here are equal to moldm-3s-1. These units are the same as the units for reaction rate.
Rate equation
Reactions can be written in a rate equation:
A + B + C → D
rate = k [A]x [B]y [C]z
Where x, y, z is the order of the reation, and k is the rate constant.
Order of a reaction
Changing concentration can change the rate of the reaction, but often different species have different rate constants
Arrhenius equation
A useful equation (given in Data Booklet) is:
k = Ae-Ea/RT
Where:
k: rate constant
Ea: activation energy
A: Arrhenius constant, it describes the geometry of reactions.
R: Gas constant = 8.31 J K−1 mol−1
T: Temperature in Kelvin
Calculating Activation Energy
By taking the natural log of the above equation:
ln k = -Ea/RT + ln A
Editors- joeClinton - 136 words.
- EightTrigrams - 264 words.
- pdumnoenchanvanit - 125 words.
View count: 13027