Measuring Energy changes
What is Energetics?
Energetics deals with heat changes in chemical reactions
Where heat is a form of energy.
Temperature: Measure of the average kinetic energy of the particles in a substance.
The first law of thermodynamics
Energy cannot be created nor destroyed, but it can be transformed from one form to another.
The second law of thermodynamics
For a reaction to occur spontaneously there must be an overall increase in entropy.
Enthalpy
Enthalpy is the amount of heat energy contained in a substance, which is stored in the chemical bonds. The enthalpy of a substance cannot be measured directly, we can only measure the change in enthalpy. The enthalpy of a substance is comprised out of kinetic and potential energy of its particles. The potential component arises from the intermolecular forces present between the particles due to their position relative to each other, whereas the kinetic component arises purely because of the particles' movement.
- It is measured in KJmol-1
- The symbol for enthalpy is
- The values are expressed under standard conditions and standard states
System vs Surrounding: The system is the reaction mixture while the surrounding is everything around it.
Important: in order to measure the enthalpy change, compounds need to be in their standard states & under standard conditions e.g. 298 K & 101 kPa & 1 mol/dm3 solution.
Standard enthalpy change of a reaction - enthalpy change when molar quantities of reactants in their standard states react to form products in their standard states under standard conditions.
What are thermochemical equations?
A thermochemical equation is a regular chemical equation but with the addition of the enthalpy change.
Note: You must remember to include state symbols.
Exothermic vs Endothermic reactions
| Reaction type |
Releases/absorbs heat |
Enthalpy sign |
Stability of products |
| Exothermic |
Releases heat energy to surroundings. |
Enthalpy is negative. |
Products more stable than reaction (lower enthalpy) |
| Endothermic |
Absorbs heat energy from surroundings |
Enthalpy is positive |
Products less stable than reactants (higher enthalpy) |
Examples of reactions that are always exothermic are combustion, neutralization, and bond formation.
Calculating enthalpy of reaction in an experiment
Q = mcΔT
- Calculate the number of moles of limiting reactant
- Calculate energy change based on the mass of water, specific heat capacity and temperature change.
- To get the enthalpy change use the following equation.
ΔH = Q/n
- ΔH = enthalpy change(kJ mol-1)
- Q = heat released(kJ) by combusting/reacting a fixed mass of a substance
- n = number of moles of a substance being combusted/reacted.
Combustion
Enthalpy of combustion is defined as energy change when 1 mole of a substance is burnt in excess oxygen.
We distinguish between complete and incomplete combustion.
| Complete combustion(excess of oxygen) |
Incomplete combustion(lack of oxygen) |
|
C3H8 + 5O2 ⇒ 3CO2 + 4H2O
|
C3H8 + 7/2O2 ⇒ 3CO + 4H2O |
| C3H8 + 2O2 ⇒ 3C + 4H2O |
The values for enthalpy of combustion can be found in section 13 of the data booklet
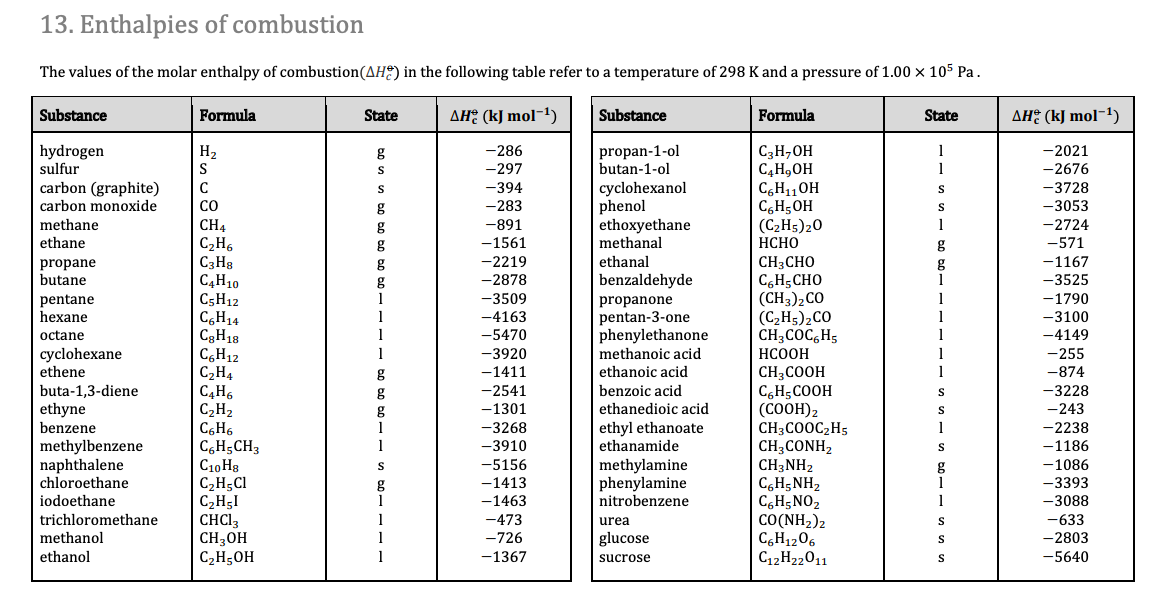
We can also notice that the magnitude of the enthalpy change increases going down the table. This is due to the greater number of carbon dioxide and water molecules being produced, as the length of the carbon chain increases.
Reasons complete combustion is usually preferable:
- It releases more energy than incomplete combustion, as all reactants are consumed during the reaction.
- Incomplete combustion can produce carbon monoxide which is poisonous.
Neutralization
Enthalpy of neutralization is defined as energy change when an acid and an alkali react to form 1 mole of water under standard conditions (IB doesn't require the definition for the enthalpy of neutralization).
Formation
Enthalpy of formation is defined as the enthalpy change when 1 mole of a compound is produced from its elements under standard conditions.
- All elements in their standard states have a 0 standard enthalpy of formation.
- Enthalpies of formation are determined using Hess's law.
- Usually, but not always exothermic.
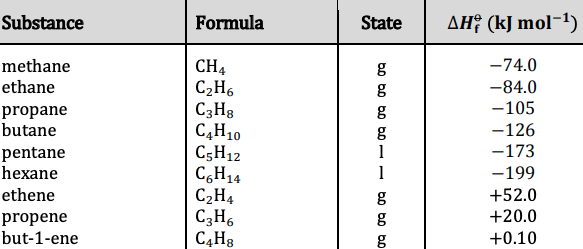
Refer to table 12 in the data booklet for relevant enthalpies of formation.
Hess's Law
Hess’s law states that the total enthalpy change for a reaction is independent of the route taken. It is a special case of the law of conservation of energy.

Enthalpy of reaction
∆Hreaction (using enthalpies of formation) = ∑∆Hf (products) – ∑∆Hf (reactants)
∆Hreaction (using enthalpies of combustion) = ∑∆Hf (reactants) – ∑∆Hf (products)
Bond Enthalpy
Bond Breaking: Endothermic process as energy is needed to separate the atoms in a bond.
Bond Making: Exothermic process as energy is released when atoms are bonded together.
- The greater the value, the stronger the bond
Enthalpy of reaction using bond enthalpies
∆H = Bond enthalpy of reactants - bond enthalpy of products.
This is because making bonds release energy (negative enthalpy) and breaking bonds takes in energy ( positive enthalpy).
Bond enthalpy values can be found in section 11 of the data booklet, but the important thing to remember is that those represent mean bond enthalpy values, which means that it is just an average. They are approximate, and might not correspond to the real value, but still can be used to calculate overall enthalpy changes.
Entropy(S)
Entropy (S) refers to a distribution of available energy among the particles in a system i.e. the more ways the energy can be distributed, the higher the entropy.
- Entropy is denoted the symbol S.
- In order of increasing entropy: solids, liquids, aqueous solutions, gases.
- Hence, going from a less disordered substance to a more disordered substance will result in an increase in entropy (e.g. going from a solid to a gas)
- Going from a more disordered substance to a less disordered substance will result in a decrease in entropy (e.g. going from a gas to a liquid)
- Absolute entropy values can be found in section 12 of the data booklet.
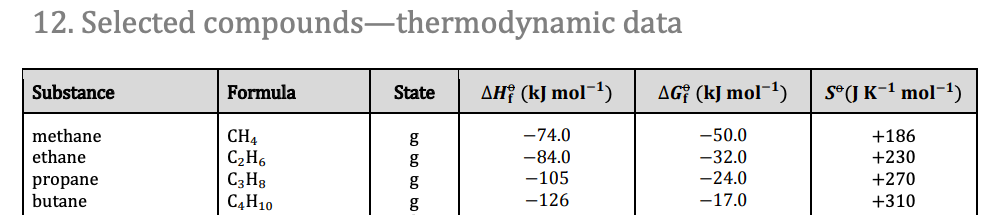
- Change in entropy can be calculated using the absolute entropy values of products and reactants.
-
If a change in entropy is positive, then the system becomes more disordered (less order). If a change in entropy is negative, then the system becomes less disordered (more order).
Gibbs free energy(G)
2nd law of thermodynamics: for a reaction to occur spontaneously there must be an overall increase in entropy.
It is an expression that links entropy and enthalpy together:
If ΔG is negative, then a reaction is spontaneous and once it starts it will continue.
Certain predictions can be made from the relative values of ΔH & ΔS.
| ΔH |
ΔS |
ΔG |
Spontaneous? |
| Positive (endothermic) |
Positive (more disorder) |
Temperature-dependent |
Yes, after reaching a certain temperature (at high temperatures) |
| Positive (endothermic) |
Negative (more order) |
Positive |
No |
| Negative (exothermic) |
Positive (more disorder) |
Negative |
Yes |
| Negative (exothermic) |
Negative (more order) |
Temperature-dependent) |
Yes, before reaching a certain temperature (at low temperatures) |
Gibbs free energy of formation can be calculated directly:
The Born-Haber cycle
Bond energies give information about molecular substances, while lattice energy measures the strength of the attraction between ions of opposite charges in crystalline solids. The Born-Haber cycle is used for those ionic compounds.
Lattice energy - enthalpy change when 1 mole of an ionic compound breaks up into gaseous ions under standard conditions.
- MN(s) → M+(g) + N-(g) + ΔHlat
Lattice energies are endothermic since bonds are broken. They allow us to compare the strength of ionic bonds. Their values can be found in section 18 of the data booklet.
Negative lattice energy is the opposite process of the formation of ions and is an exothermic process.
- M+(g) + N-(g) → MN(s) - ΔHlat
Quite ambiguously, lattice energies can be used for completely opposite processes: the formation of an ionic compound (exothermic) and the dissociation of an ionic compound (endothermic). The IB only considers the latter definition.

Above, you can see the diagram that can help calculate the lattice energy of LiF. We want Li+(g) + F-(s) → LiF(s):
- Convert Li to Li+
- Convert solid Na into 1 mole of free atoms in gaseous states (enthalpy of vaporization on the diagram): Na(s) → Na(g). This is called atomization, and the value for ΔHatom is usually given in the questions.
- Ionize Li (ionization enthalpy on the diagram): Li(g) → Li+(g). It requires using the value for first ionization energy, which can be found in section 8 of the data booklet. Note: if in other examples, the element form 2+, 3+, etc. ions, you are required to add the consecutive ionization energies. Ex: for Mg2+ you would need to use both 1st and 2nd IEs, and then add them together.
- Convert 1/2F2 to F-
- Convert solid fluorine into 1 mole of free atoms in gaseous state (bond enthalpy(F-F) on the diagram, as you are breaking bonds in the process): 1/2F2(g)→ F-(g). This is also called automatization or bond dissociation, which is why you are required to use section 11 of the data booklet to get the value for the corresponding bond enthalpies, and then multiply it by the number of moles (coefficient before the element symbol) to get ΔHatom.
- Convert gaseous F atoms into 1 mole of gaseous ions in gaseous states(electron affinity on the diagram): F(g) + e- → F-(g). Now, you are required to use the electron affinity values, which can be found in section 8 of the data booklet. Note: if the ion has a greater charge than 1-, then you are supposed to use consecutive electron affinity values. Ex: for O2-, use 1st and 2nd EAs, and then add them together.
- From the shown cycle it can be stated:
- Note: enthalpy of formation can be found in section 12 of the data booklet.
What is the whole point of lattice enthalpies?
Since it is a measure of the strength of the electrostatic attraction between ions of opposite charges, its value mostly depends upon 2 factors:
- Ionic charge
- Ionic radii
- The smaller the ionic radius, the shorter the distance between the ions and hence the stronger the attraction between them.
Editors- joeClinton - 287 words.
- Bill Wowden - 30 words.
- polina.blinova - 1348 words.
View count: 35885