Disclaimer: This only covers the SL information, if you are in HL feel free to edit the page to add the HL content.
Ionic bonding
Ions
Ions are elements which have had electrons added or removed, giving them either a positive or negative charge.
Cation vs Anion
Cation: An ion with fewer electrons than normally. It has a positive charge.
Anion: An ion with more electrons than normally. It has a negative charge.
Ionic compounds
- Most often between a non-metal and metal
- These consist of ions held together in a lattice structure via electrostatic attraction
- The formula for an ionic compound is expressed in its simplest ratios. E.g
Coordination number: The number of ions surrounding each ion in the lattice
Properties of Ionic Compounds
They are electrically neutral, meaning they have a net charge of zero. This is due to the fact that for an ionic bond between two ions to occur, the magnitude of the negative charge of one ion must be equal to the magnitude of the positive charge of the other ion. So when combined, they cancel out. However, ionic bonds will always have a dipole, due to the large difference in electronegativity of the two bonding substances.
High melting points and boiling points. This is due to the fact that the distribution of charge across the ion isn't uniform, as the bond is polar. This means they can form dipole-dipole bonds, a type of intermolecular bond. This intermolecular bond means more energy is required to overcome the potential energy well of the bonds.
More soluble in polar solvents (E.g water) than non-polar solvents. This is because ionically bonded substances themselves are polar, so they can disassociate in polar solvents.
Conduct electricity when dissolved in an aqueous solution because the ions disassociate. This means the negatively charged and positively charged ions separate when dissolved. When a potential difference is applied to this solution, these ions allow for easy flow of charge.
Diagram of Ionic bond

Covalent bonding and structures
What is a covalent bond?
This is the electrostatic attraction between the positively charged nuclei and a pair negatively charged electrons
Double and triple bonds
Double bond: Two pairs of shared electrons
Triple bond: Three pairs of shared electrons
The more bonds the stronger and shorter the bond
Lewis structures
A Lewis structure is a diagram specifically for Covalent bonding Which shows which valence electrons are shared in the bond of a molecule.
How to draw it:
- Start by drawing all the electrons as crosses around an element
- Then circle electron pairs to show that they are shared across the elements.
- Circle the electrons so that they add up to 8; a full shell.
- Neaten it up by replacing any pairs of electrons with a single line
Example diagram
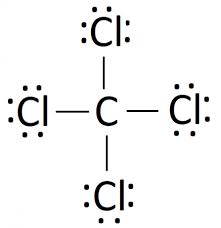
Octet Rule
Most atoms want to form a stable outer shell with eight electrons
Exceptions:
Less than an octet: There can be less than an octet in the outer shell if the central atom is very small. This is because it does not require many electrons to balance the weak inner charge. E.g BeCl2
Expanded octet: There can be more than an octet in the outer shell if the central atom is very big. This is because it does not require many electrons to balance the weak inner charge. E.g SF6
VSEPR Theory
VSEPR: Valence shell electron pair repulsion
Electron domain: A dense area of electrons. Both single double and triple are counted as one electron domain.
- The geometrical arrangement is determined by the total number of electron domains by the maximum repulsion.
- The shape of the molecule is determined by the number of bonding pairs in this arrangement
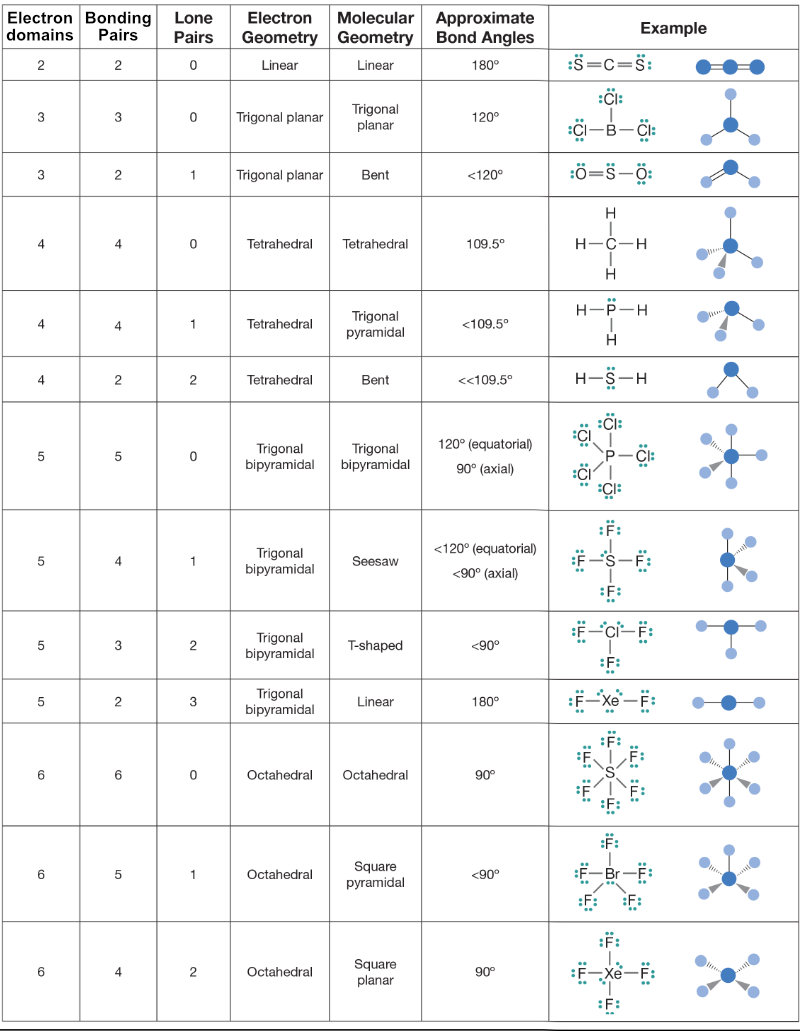
Resonance structures
These occur when there is more than one possible location for a double or triple bond.
Bond order: This is the average number of bonds making up an electron domain. In the case of resonance structures, this can be a decimal like 1.5
Intermolecular forces
Types of IMF's
| IMF type |
What causes it |
What factors affect it |
| London Dispersion |
Temporary electron dense areas make an atom have a dipole. Then this dipole induces a dipole in a nearby atom, which repeats the cycle. |
- Ease of electrons to form dipoles
- The surface area of molecules as there are more attractions
- Mass of the compound as there will be more electrons to shift and create temporary dipoles.
|
| Dipole-Dipole |
Polar and non-polar sides of molecules attract due to electrostatic attraction |
- Electronegativity difference
- Polarity
|
| Hydrogen bonding |
Occurs when hydrogen is bonded to a strongly electronegative element. (Nitrogen, oxygen or fluorine) A stronger version of dipole-dipole bonding, due to large electronegativity difference.
The electronegative element has a lone pair of electrons, so is very negative. Hydrogen is positively charged. They are attracted.
|
- Temperature as hydrogen bonds breaks apart at high temperature.
|
Affect of hydrogen bonding on boiling/melting point
Molecules that form hydrogen bonds will have a much larger boiling point than periodic trends would suggest.
This is due to the strength of hydrogen bonds compared to dipole-dipole bonds. This shown in the graph below.

Metallic Bonding
What are the Metals?
We use metals often in our everyday lives, in wires, in goalposts and in oven grids. But chemically, what defines a metal? What separates it from other elements? Metals are elements who's highest energy valence electrons lie in the d block of the periodic table (when atoms). They are in groups 3-12 on the periodic table and are over 70% of known elements. Metals elements (alloys do not necessarily have these properties) share many similar properties. They are shiny, they are (almost all) solid at room temperature. They are also malleable and ductile, meaning they can be bent and stretched with some force applied. They also conduct electricity and heat really well.
How does metallic bonding work?
 Figure 1
Figure 1
Metallic bonding is based upon the electrostatic attraction between the positively charged cations, and the negatively charged delocalised electrons (delocalised means that they aren't attached to the nucleus). In the diagram above, the + represent the metal cations, and they represent the delocalised electrons. The attraction between cations and electrons is what makes the metal structure. Although initially, the electrons must have come from one of the ions, the initial donor of the electron doesn't matter, all electrons behave in the same way. They are delocalised, meaning they aren't part of the ion anymore.
Metals form a giant, uniform lattice. The lattice expands outwards in all directions (3d), unlike graphite for example, which extends outwards in a flat plane. This means that there are no individual bonds, just a giant network of cations and electrons. Additionally, assuming it's just one metal (it's different with alloys), the structure does not change throughout the network and is uniform.
What affects strength of metallic bond
- Number of delocalised electrons
- Charge on the metal ion
- Ion size (small has a stronger bond)
Properties of Metallic bonding
Conductivity of electricity
Metals are highly conductive, meaning they conduct electricity well. Electric current is essentially just the flow of charge in a certain direction. As the electrons in the lattice are delocalised, that means they can easily move. Additionally, electrons have a charge of -1, meaning if they move in a certain direction, there is a flow of charge. To get these electrons to move in one direction, a potential difference (voltage) must be applied to the lattice. Electrons are repelled from the negative electrode and move towards the negative electrode, creating a flow of charge across the metal lattice.
Conductivity of heat
Metals can also conduct heat. This means that heat flows through metal with ease. Heat is just kinetic energy. The delocalised electrons with high kinetic energy vibrate, distributing their kinetic energy around the lattice. Because of entropy, over time the energy will be distributed evenly across the lattice. Note that the electrons don't themselves don't move around the lattice, it's more like a wave, a constant transfer of energy across the lattice.
Ductility and Malleability
Metals are ductile and malleable because of the uniformity of the giant lattice. Now, this provokes the question, why aren't giant covalent structures like diamond malleable or ductile. The reason for this is that in a giant covalent structure, every particle is chemically bonded to a nearby particle, so to slide one layer over another requires breaking the bonds, applying a force and then forming the bond again. But in a metallic structure, electrostatic force holds the lattice together. To slide one layer over another, some force is required to overcome the electrostatic attraction. However, no bonds need to be broken, and there (in theory) is no reduction in structural integrity, because of the uniformity of the structure.
Editors- joeClinton - 713 words.
- Bill Z - 4 words.
- EightTrigrams - 728 words.
- CD_FER - 1 word.
View count: 21705